The Ph of a Solution Is Defined as
Calculate the concentration of hydrogen ions in moles per liter M. The term widely used in chemistry biology and agronomy translates the values of the concentration of the hydrogen ion which ordinarily ranges between about 1 and 10 14 gram-equivalents per litreinto numbers between 0 and 14.
PH is the negative base 10 logarithm log on a calculator of the hydrogen ion concentration of a solution.

. The pH scale pH is a numeric scale which is used to define how acidic or basic an aqueous solution is. The solute is the substance that is dissolved in the solvent. The pH to H formula that represents this relation is.
Fill in the table below. H M B The pH of lemon juice is 20. The pH of a solution is also defined as the logarithm of the reciprocal of left rm H right ion concentration.
Aqueous solutions at 25C with a pH less than 7 are acidic while those with a pH greater than 7 are basic or alkaline. H hydrogen ion concentration a Use the rules for logarithms and exponents to solve for H in terms of pH Submit Submit Answer Retry Entire Group 4 more group attempts remaining The pK of a solution is defined by the equation. The range of the pH scale varies from 0 to 14.
The molarity of the given formic acid solution whose K a is 17 10 4 and pH is 326 at 25 C. Likewise the hydroxide ion molarity may be expressed as a p-function. 4 The pH of a solution is defined by the equation.
Specifically pH is the negative logarithm of hydrogen ion H concentration molL in an aqueous solution. The pH of a solution is defined as the logarithm of the reciprocal of the hydrogen ion concentration or activity H of the solution. A solution consists of a solute and a solvent.
PH is logarithmically and inversely related to the concentration of hydrogen ions in a solution. The pH is a measure of the concentration of hydrogen ions the acidity or alkalinity of a solution. Aqueous solutions at 25 C with a pH of less than 7 are acidic and basic or alkaline solutions are those with a pH greater than 7.
H 3 O 10 pH. Even though pH is a unitless value but it is not a random scale. Beer has a pH of 3 therefore its H concentration is ___ times greater than a solution with a pH of 5.
PH -log H Where. The pH scale runs from 0 to 14a value of seven is considered neutral less than seven acidic and greater than seven basic. PH -log 10 H.
Of a solution is defined as the. Chemistry questions and answers. Bases added to water.
PH is a measure of the amount of hydrogen ion activity H in a solution and therefore its alkalinity or acidity. The pH of a solution is defined as the negative base-10 logarithm of the hydrogen or hydronium ion concentration. As a pH of a solution increases the concentration of ______ ions increases.
The meaning of PH is a measure of acidity and alkalinity of a solution that is a number on a scale on which a value of 7 represents neutrality and lower numbers indicate increasing acidity and higher numbers increasing alkalinity and on which each unit of change represents a tenfold change in acidity or alkalinity and that is the negative logarithm of the effective hydrogen-ion. A solution is a homogeneous mixture of two or more substances. PH -logH Values of pH.
The pH of a solution is defined as the _______ _______ of the hydrogen ion concentration. PH in other words is a scale that is used to specify the acidity or basicity of an aqueous solution. The pH scale for acidity is defined by pH log10 H where H is the concentration of hydrogen ions measured in moles per liter M.
2711pHlogHBecause the ion product of water Kw HOH 104 1014 at 25C it follows that a neutral solution eg pure water at 25C in which HOH has a pH7. PH -log 10 H The term is used to indicate basicity or acidity of a solution on a scale of 0 to 14 with pH 7 being neutral. PH Formula is expressed as.
PH is a measure of hydrogen ion concentration a measure of the acidity or alkalinity of a solution. Calculate the concentration of hydrogen ions in moles per liter M. Negative logarithm of the magnitude of hydrogen ion concentration.
Compounds that increase the concentration of negatively charged hydroxyl ions when dissolved in water. The pH of a solution is therefore defined as shown here where H 3 O is the molar concentration of hydronium ion in the solution. PH -logH To Calculate.
Causes the formation of additional hydronium ions and a resulting lower concentration of hydroxyl ions. PH is a measure of how acidic or basic a chemical solution is. The measure of acidity or alkalinity of a solution.
A The pH of egg whites is 83. The value is due to the activity of H ion in the solution. PK -log K Where.
PH logH 3 O Rearranging this equation to isolate the hydronium ion molarity yields the equivalent expression. The pH scale usually ranges from 0 to 14. Following is the equation that is used for calculating the pH.
PH is defined as the measure of hydrogen ion concentration used to measure the acidity or alkalinity of a given solution. As the concentration of H ions in solution increases acidity increases and pH gets lower below 7 see. When we talk about the pH potential of hydrogen or power of hydrogen of a solution we are basically discussing the measure of hydrogen ion concentration in a solution.
PH quantitative measure of the acidity or basicity of aqueous or other liquid solutions. The pH of a solution is the negative logarithm to the base 10 of the hydrogen ion concentration expressed in moles per litre. Logarithm of reciprocal of magnitude of hydrogen ion concentration.
In chemistry the pH of a solution is defined by the equation pH -logH where H represents the concentration of hydrogen ions in the solution. Any solution with a pH less than 7 is considered acidic and any solution with a pH greater than 7 is considered basic. Acid added to water.
The pH of a solution is defined as the A negative logarithm class 12 chemistry CBSE. PH is described as the negative of the logarithm of the molar hydronium-ion concentration. A solution may exist in any phase.
Updated on May 07 2019. PH is an abbreviation for power of hydrogen where p is short for the potenz the German word for power and H is the symbol of the element hydrogen. The pH-scale is normally between 0 and 14.
It commonly ranges between 0 and 14 but can go beyond these values if sufficiently acidicbasic. Round your pHs to the nearest tenth of a unit. The amount of solute that can be dissolved in solvent is called its solubility.

Thesis Dissertation Writing Help In The Uk Finding Reliable Solution Digital World Phdassistance Dissertation Writing Thesis Writing Writing Help

Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Solutions Buffer

The Scale Of Ph Physical Chemistry Negative Exponents Dissociation
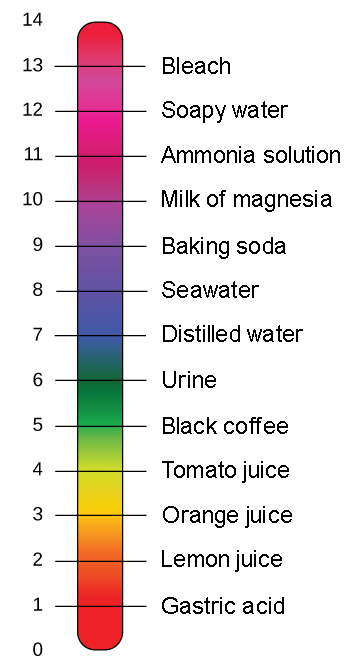
Buffers Ph Acids And Bases Biology For Non Majors I

Ph Scale Soil Ph Alkaline Alkaline Diet

The Ph Scale Chemistry For Non Majors
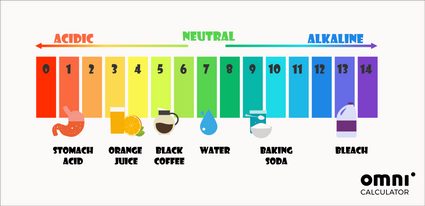
Ph Calculator How To Calculate Ph

Determining Ph Methods Classification Video Lesson Transcript Study Com

Factor Affecting Uv Vis Absorption Lalit Buffer Solution Factors Reaction Rate

Ph Of A Solution An Overview Sciencedirect Topics

Ph Of A Solution An Overview Sciencedirect Topics

The Best Organic Fertilizers To Double Your Harvest Hydroponic Nutrient Solution Healthy Garden Soil Healthy Garden







Comments
Post a Comment